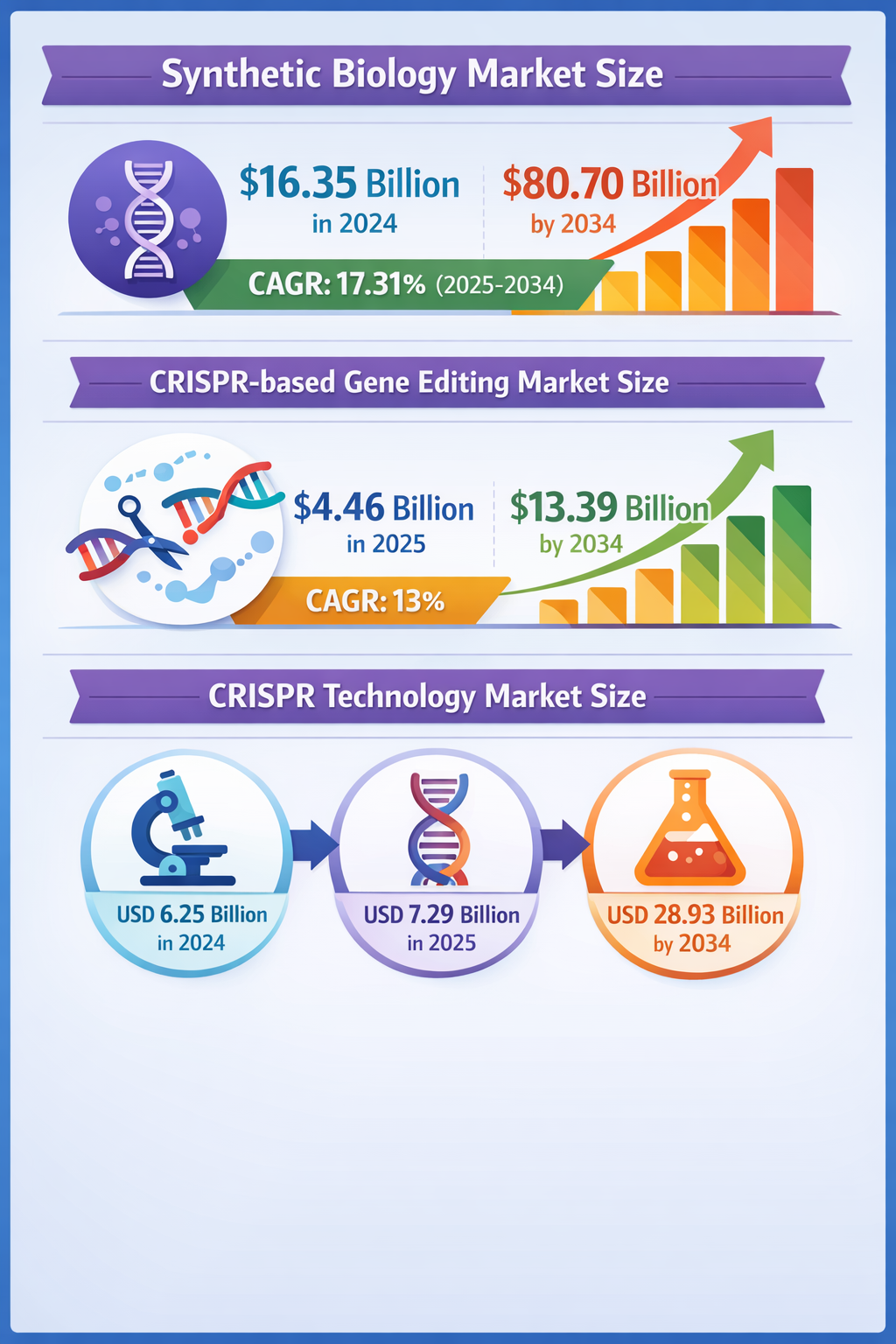
The Emerging Potential of Accelerated Gene-Editing Regulation to Disrupt Healthcare and Beyond
Recent regulatory shifts hint at a significant acceleration in the approval process for gene-editing therapies, particularly those using CRISPR technology. This weak signal of faster, more flexible regulatory pathways could trigger a wave of innovation and industrial transformation far beyond rare diseases, fundamentally altering healthcare delivery, biomanufacturing, and synthetic biology applications within the next decade.
What’s Changing?
In 2025, the U.S. Food and Drug Administration (FDA) announced plans to unveil a faster approval process specifically designed for custom gene-editing treatments (FDA announcement via Financial Post). This initiative aims to catalyze industry investment by reducing the timeline from discovery to clinical deployment, especially for therapies targeting rare diseases.
Concurrent advances in gene editing, largely driven by CRISPR-based platforms, show clinical breakthroughs on the horizon. The first wave of approvals for diseases like sickle cell anemia may arrive as early as 2025-2026 (Intuition Labs), potentially jumpstarting an entirely new biotechnology subindustry focused on in vivo gene corrections. These developments could redefine drug development logistics and healthcare economics.
Beyond rare diseases, gene editing and cell therapies are expected to expand into common conditions by 2028, signaling a shift from niche to mainstream applications (Ian Khan). This scale-up will likely demand novel delivery platforms, such as CRISPR Therapeutics’ lipid nanoparticle technology for safe and precise in vivo edits (Investing News).
China’s strategic prioritization of synthetic biology and gene editing within its Five-Year Plans (PMC Article) suggests a global race to lead next-generation biotechnologies. This political will combined with accelerated regulatory strategies may usher in a new era of bioeconomy competition with worldwide implications.
In parallel, synthetic biology is poised to drastically reduce reliance on traditional agriculture by enabling more efficient food production techniques, including engineered microbes and lab-grown meat (The Conversation, Ian Khan). This serves to illustrate gene editing’s implications far beyond therapeutics, expanding into climate resilience and food security sectors.
Why Is This Important?
The acceleration of gene-editing therapy approvals could disrupt regulatory inertia that has slowed drug innovation for decades. It may open investment floodgates, allowing smaller startups and new entrants to compete with established pharmaceutical firms, thereby democratizing innovation.
By lowering barriers for clinical trials and patient access, these changes could shorten the timeline to market for therapies targeting both rare and common diseases. This could significantly impact patient outcomes, healthcare insurance models, and public health strategies globally.
Furthermore, the entry of gene editing into everyday medicine might force industries such as agriculture, environmental management, and manufacturing to adapt rapidly as bioengineering solutions become viable alternatives to conventional processes.
The geopolitical implications are notable as innovation races intensify between countries prioritizing advanced biotechnology, potentially influencing global supply chains, intellectual property landscapes, and international regulatory harmonization efforts.
Implications
For pharmaceutical companies and biotech investors, the evolving regulatory environment demands closer scrutiny of emerging gene-editing platforms and early-stage candidates. Agile engagement with regulators and investment in delivery technologies may be essential for competitive positioning.
Healthcare systems may need to prepare for a paradigm shift in patient care models, integrating gene therapies into standard protocols and adjusting reimbursement frameworks accordingly. This could also prompt ethical and societal debates around accessibility and long-term safety monitoring.
Governments and policymakers might consider balancing accelerated approvals with robust post-market surveillance to mitigate risks associated with new gene-editing modalities. Strategic international collaborations could improve cross-border innovation while ensuring biosafety and equitable access.
In sectors like agriculture and food production, synthetic biology innovations fueled by more flexible regulations could lead to disruptive business models. Traditional suppliers may face existential challenges as engineered proteins and microbial systems reduce the need for farmland and livestock.
Finally, workforce planning across industries must anticipate new skill sets in genetic engineering, bioinformatics, and clinical gene therapy implementation, highlighting opportunities for reskilling and workforce development.
Questions
- How can organizations position themselves to leverage expedited regulatory pathways for gene editing without compromising safety or public trust?
- What collaborative frameworks could governments and industry establish to promote responsible innovation and global harmonization in gene-editing approvals?
- How might accelerated gene-editing therapies transform insurance, reimbursement, and healthcare delivery models over the next 5 to 10 years?
- What are the potential cascading economic impacts on traditional industries like agriculture and pharmaceuticals as synthetic biology products mature?
- Which ethical considerations might grow as gene-editing therapies expand beyond rare diseases to broader populations?
- How should workforce development strategies adapt to prepare for the anticipated surge in demand for gene-editing expertise?
Keywords
gene editing; CRISPR; accelerated regulation; synthetic biology; biotechnology investment; healthcare disruption; food security; global bioeconomy
Bibliography
- A top United States regulator plans to unveil a faster approach to approving custom gene-editing treatments, a move designed to unleash a wave of industry investment that will yield cures for patients with rare diseases. Financial Post. https://financialpost.com/technology/fda-gene-editing-therapy
- Breakthroughs in science will create new hiring spurts: for example, if CRISPR-based gene therapies begin to succeed clinically (the first approvals could happen around 2025-2026 for diseases like sickle cell), an entire subindustry of gene editing therapies could blossom. Intuition Labs. https://intuitionlabs.ai/articles/us-biotech-job-market-2025
- Venture capital investment in cellular agriculture will exceed $50 billion over the next five years, driving accelerated innovation and scaling. Ian Khan. https://www.iankhan.com/lab-grown-meat-in-2035-my-predictions-as-a-technology-futurist-7/
- Synthetic biology could even drastically reduce how much farmland the world needs by producing food more efficiently. The Conversation. https://theconversation.com/engineered-microbes-could-tackle-climate-change-if-we-ensure-its-done-safely-266584
- CRISPR Therapeutics is advancing a pipeline of in vivo gene editing candidates addressing major unmet needs in cardiovascular, metabolic and rare diseases using its proprietary, de-risked lipid nanoparticle delivery platform. Investing News. https://investingnews.com/crispr-therapeutics-provides-business-update-and-reports-third-quarter-2025-financial-results/
- China has placed significant emphasis on synthetic biology and gene editing technologies, making them strategic priorities in the 13th and 14th Five-Year Plans. PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC12479303/